Reprogramming Stem Cells to Create Retinal Sheets: A Promising Approach to Restoring Vision
A groundbreaking study by researchers from the University of Montreal, led by Professor Gilbert Bernier, has demonstrated that retinal transplants derived from stem cells can restore vision in blind minipigs. Published in the journal Development, the study represents a significant step toward developing treatments for retinal degenerative diseases.
Developing Retinal Sheets from Stem Cells
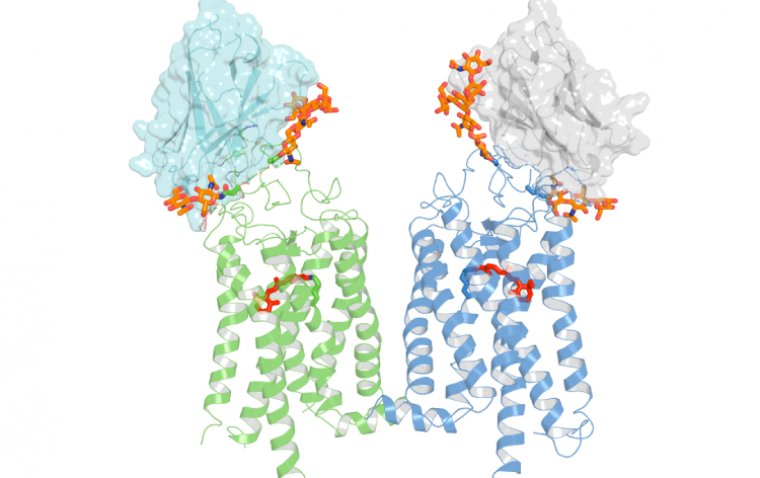
Professor Bernier’s team successfully developed a method to reprogram human induced pluripotent stem cells (hiPSCs) into structured sheets of retinal cells. These stem cells, reprogrammed from mature adult cells, have the unique ability to differentiate into any cell type in the body.
The researchers focused on generating "retinal sheets" enriched with immature cone photoreceptor cells. These cone cells, essential for color vision and sharp central vision, matured in laboratory cultures, mimicking the natural retinal structure.
Transplanting Retinal Sheets into Minipigs
After successfully engineering the retinal sheets in vitro, the next challenge was transplantation. The team chose minipigs as their model due to the similarity of their eye size and weight to humans, allowing retinal surgeons to perform procedures that closely resemble potential human applications.
Upon transplantation, the retinal grafts integrated into the minipigs' damaged retinal tissue. Encouragingly, the minipigs exhibited signs of vision restoration. The researchers observed the formation of new neural connections between the transplanted photoreceptor cells and the host neural cells. Additionally, when exposed to light, the grafted area showed neural activity, suggesting functional vision improvement.
Addressing the Challenges of Retinal Regeneration
Given the urgent need for effective treatments against vision loss, researchers worldwide are exploring various approaches to repairing damaged macula.
"Some approaches use dissociated photoreceptor cells; others create micro-dissected retinal organoids, which are lab-grown 'mini-organs' in a dish," explains Professor Bernier.
However, his team’s method offers a distinct advantage: it enables the spontaneous formation of flat retinal tissue that is pre-organized and polarized, closely resembling the structure of the human embryonic retina. This method also allows the generation of large quantities of retinal tissue for transplantation.
Surgical Limitations and Future Directions
One of the key challenges in this approach is ensuring precise placement and orientation of the grafts during surgery. The macula, a critical region for central vision, measures only about 4mm in diameter—approximately the size of a grain of rice.
"To properly orient, place, and stabilize the graft in the retina remains a significant surgical challenge," says Professor Bernier.
To improve transplantation success, the team is developing and validating an experimental retinal surgery device designed to optimize the placement and stability of the grafts.
A Step Closer to Treating Retinal Degenerative Diseases
Despite existing challenges, this study highlights the potential of retinal sheet transplantation as a promising strategy for treating retinal degenerative conditions. The findings pave the way for future advancements in stem cell-based therapies, bringing hope to individuals affected by vision loss.
Reference:
Andrea Barabino et al, Molecular characterization and sub-retinal transplantation of hypoimmunogenic human retinal sheets in a minipig model of severe photoreceptor degeneration, Development (2024). DOI: 10.1242/dev.203071
(1).jpg)