Stanford Study Reveals Ocular Side Effects Linked to Ovarian Cancer Drug
A recent study presented at the American Academy of Ophthalmology (AAO) meeting in Chicago has identified significant ocular side effects associated with mirvetuximab soravtansine (Elahere), a drug approved for ovarian cancer treatment. The research, conducted by Stanford University, highlights the need for close ophthalmic monitoring of patients receiving this therapy.
Mirvetuximab and Corneal Toxicity: Key Findings
Mirvetuximab soravtansine belongs to a new class of anti-cancer drugs known as antibody-drug conjugates. Approved by the FDA in 2022 for ovarian cancer, the drug's label included warnings about potential ocular side effects. However, real-world data on these adverse effects were previously lacking. The Stanford Ophthalmology team, led by Dr. Prithvi Mruthyunjaya, in collaboration with the Department of Obstetrics and Gynecology, conducted a study to investigate this drug’s impact on the eyes.
The study followed 18 women (36 eyes) receiving mirvetuximab treatment for approximately six months. The findings revealed that:
• 47% of eyes experienced moderate or severe corneal toxicity.
• 22% of eyes displayed mild toxicity.
• 31% of eyes remained unaffected by the treatment.
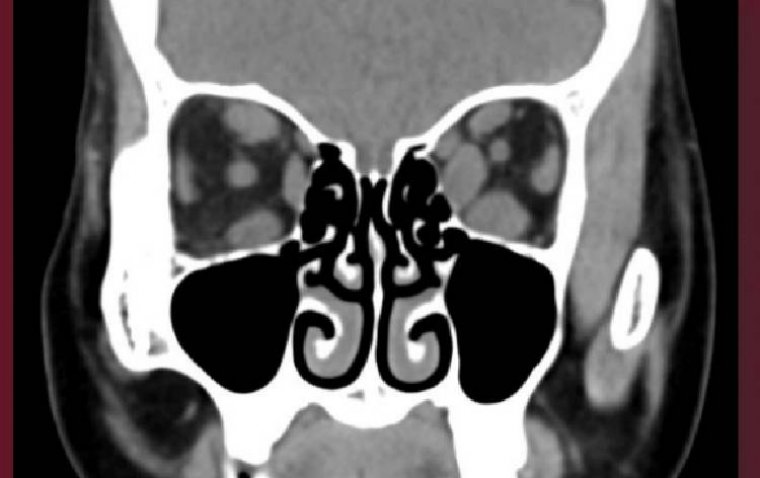
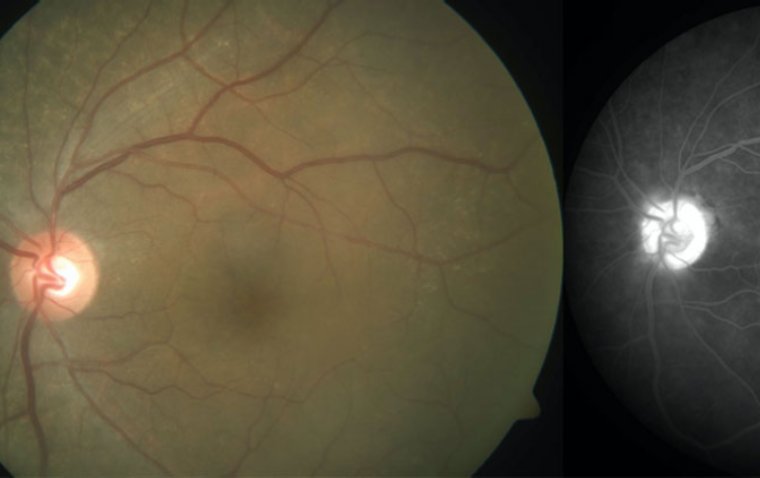
The most prevalent corneal issues were paracentral ring-like subepithelial cystic deposits, corneal haze, and filamentous keratitis, which led to symptoms such as blurry vision, eye pain, light sensitivity, and foreign body sensation. Notably, over half of the patients in the study experienced decreased vision as a result of these side effects.
Management and Resolution of Ocular Toxicity
The study found that most women experienced improvement or complete resolution of symptoms following a regimen of high-potency topical corticosteroids combined with a reduced dosage or frequency of mirvetuximab infusions. By the last follow-up visit:
• 65% of eyes had returned to baseline vision.
• Corneal toxicity had significantly resolved in nearly all patients.
Lead researcher Dr. Filippos Vingopoulos emphasized the importance of vigilant ophthalmic follow-up for oncology patients on mirvetuximab. “Close ophthalmology follow-up is required for oncology patients on mirvetuximab, as high-potency topical corticosteroids along with dose reduction or spacing might be needed to preserve or restore vision,” he stated in the AAO press release.
Recommendations for Ophthalmic Monitoring
In light of these findings, experts recommend baseline eye examinations for patients beginning mirvetuximab therapy, followed by routine exams at least every other cycle for the first eight cycles. This proactive approach can help detect and manage ocular toxicity early, preventing more severe complications.
Collaboration for Optimal Patient Care
Dr. Vingopoulos also stressed the importance of multidisciplinary collaboration in managing side effects of novel cancer therapies. He noted, “As novel promising treatments are incorporated into our anti-cancer treatment algorithms, post-market surveillance with real-world studies and close collaboration between ophthalmology and multiple other disciplines of medicine will allow for optimal care for our patients.”
Conclusion
The Stanford University study underscores the significance of post-market surveillance and interdepartmental cooperation in managing the ocular side effects of new cancer drugs like mirvetuximab soravtansine. By prioritizing regular eye exams and proactive treatment, healthcare providers can better protect the vision of oncology patients receiving these therapies.
Source:
(1).jpg)