Neuroscientists Discover a Small Molecule That Restores Visual Function After Optic Nerve Injury
Traumatic injury to the central nervous system (CNS), spinal cord, and optic nerve is the primary cause of disability and the second largest cause of mortality worldwide. CNS injuries frequently result in catastrophic loss of sensory, motor, and visual skills, which is the most difficult problem for physicians and researchers.
City University of Hong Kong (CityU) neuroscientists recently identified and demonstrated a small molecule that can effectively stimulate nerve regeneration and restore visual functions following optic nerve injury, providing great hope for patients suffering from optic nerve injury, such as glaucoma-related vision loss.
"There is currently no effective treatment available for traumatic injuries to the CNS, so there is an immediate need for potential drug to promote CNS repair and ultimately achieve full function recovery, such as visual function, in patients," said Dr. Eddie Ma Chi-him, Associate Head and Associate Professor in the Department of Neuroscience and Director of the Laboratory Animal Research Unit at CityU, who led the research.
Mitochondrial Dynamics and Motility: A Critical Piece in the Puzzle of Axon Regeneration
Axons, which are a cable-like structure that extends from neurons, are responsible for carrying messages between neurons and from the brain to muscles and glands. The formation of active growth cones and the activation of a regrowth program including the synthesis and transport of materials to regrow axons is the first step toward effective axon regeneration. These are all energy-intensive activities that necessitate the active transport of mitochondria (the cell's powerhouse) to wounded axons at the distal end.
Injured neurons, therefore, face special problems that require long-distance transport of mitochondria from the soma (cell body) to distant regenerating axons, where axonal mitochondria in adults are usually immobile and local energy consumption is crucial for axon regeneration.
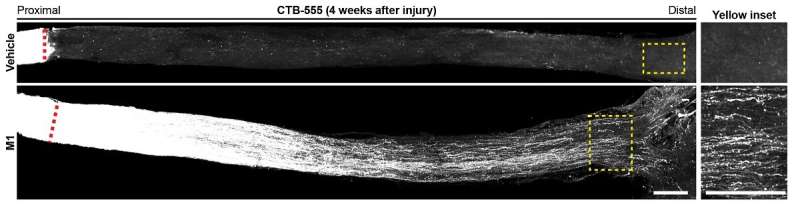
M1 induces sustained axon regeneration that reaches the optic chiasm four weeks after optic nerve crush (below). In vehicle-treated mice, virtually no regenerating axons were observed (top). Credit: Au, N. et al.
Dr. Ma's team discovered M1, a therapeutic small chemical that can accelerate mitochondrial fusion and motility, resulting in long-distance axon regeneration. In M1-treated mice, regenerated axons evoked neural activities in target brain areas and recovered visual functions four to six weeks after optic nerve injury.
M1: A Promising Small Molecule for Sustaining Long-Distance Axon Regeneration Through Mitochondrial Dynamics
"Photoreceptors in the eyes [retina] forward visual information to neurons in the retina. To facilitate the recovery of visual function after injury, the axons of the neurons must regenerate through the optic nerve and relay nerve impulses to visual targets in the brain via the optic nerve for image processing and formation," explained Dr. Ma.
The researchers investigated the degree of axon regeneration in M1-treated animals four weeks after damage to see if M1 could promote long-distance axon regeneration following CNS trauma. Surprisingly, the majority of regenerating axons in M1-treated mice reached 4mm distant to the crush site (near the optic chiasm), but no regenerating axons were observed in vehicle-treated control mice.
"This indicates that the M1 treatment sustains long-distance axon regeneration from the optic chiasm, i.e. midway between the eyes and target brain region, to multiple subcortical visual targets in the brain. Regenerated axons elicit neural activities in target brain regions and restore visual functions after M1 treatment," Dr. Ma added.
M1 Treatment Restores Visual Function
To further explore whether M1 treatment can restore visual function, the researchers gave the M1-treated mice a pupillary light reflex test six weeks after the optic nerve injury. They found that the lesioned eyes of M1-treated mice restored the pupil constriction response upon blue light illumination to a level similar to that of non-lesioned eyes, implying that M1 treatment can restore the pupil constriction response following optic nerve damage.
Furthermore, the researchers analyzed the mice's response to a looming stimulus—a visually generated natural defensive response to escape predators. The mice were placed in an open space with a triangular prism-shaped shelter and a rapidly expanding overhead-black circle to act as a looming stimulus, and their freeze and escape behaviors were examined.
Half of the M1-treated mice responded to the stimulus by hiding in a shelter, demonstrating that M1 caused robust axon regeneration to reinnervate subcortical visual target brain areas for complete visual function recovery.
Potential Clinical Application of M1 for Repairing Nervous System Injury
The seven-year study, which builds on the team's prior research on peripheral nerve regeneration using gene therapy, underlines the possibility of a readily available, non-viral therapy for CNS repair.
"This time we used the small molecule, M1, to repair the CNS simply by intravitreal injection into the eyes, which is an established medical procedure for patients, e.g. for macular degeneration treatment. Successful restoration of visual functions, such as pupillary light reflex and response to looming visual stimuli was observed in M1-treated mice four to six weeks after the optic nerve had been damaged," said Dr. Au Ngan-pan, Research Associate in the Department of Neuroscience.
The team is also working on an animal model to treat glaucoma-related vision loss, as well as other prevalent eye disorders and vision impairments include diabetes-related retinopathy, macular degeneration, and traumatic optic neuropathy. As a result, more research is needed to assess M1's potential clinical applicability.
"This research breakthrough heralds a new approach that could address unmet medical needs in accelerating functional recovery within a limited therapeutic time window after CNS injuries," said Dr. Ma.
The research was published in the journal Proceedings of the National Academy of Sciences (PNAS) as "A small molecule M1 promotes optic nerve regeneration to restore target-specific neural activity and visual function."
Reference
Ngan Pan Bennett Au et al, A small molecule M1 promotes optic nerve regeneration to restore target-specific neural activity and visual function, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2121273119
(1).jpg)