Regeneron Wins Patent Case Against Novartis for Its Eye Drug Eylea
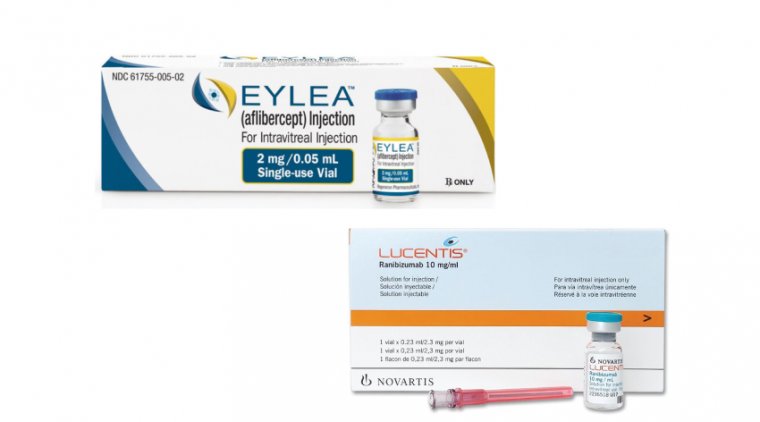
Regeneron Pharmaceuticals Inc. has won a patent case against Novartis AG in a U.S. court, protecting the company's drug delivery system for its eye drug Eylea.
The US Patent Trial and Appeal Board ruled in favor of Regeneron, stating that Novartis' pre-filled syringe for injecting its eye medication Lucentis was “unpatentable”. Eylea, a treatment for age-related macular degeneration, generates billions of dollars in annual sales for Regeneron.
In its initial complaint to the US International Trade Commission in 2020, Novartis claimed that some pre-filled intravitreal injectable syringes and, ultimately, Regeneron's delivery system Eylea, violated the company's patent. In 2021, Regeneron submitted a request for a reconsideration of Novartis' claims.
The disputed patent, designated as ‘631, which relates to a syringe for into-the-eye injections, had multiple purported foreign priority applications” and specifics regarding the syringe and the therapeutic delivery. Regeneron contested each of the 26 claims that were a part of the patent litigation.
The case observed several factors, including the “ordinary skill in the art” threshold, commercial success and prior failures. In the end, the Patent Trial and Appeal Board declared that all claims for the ‘631 patent are “unpatentable.”
This removes a roadblock for one of Regeneron's major sellers. According to Regeneron's Q3 sales figures, Eylea generated more than $1.6 billion in revenue. The FDA also extended Eylea's "pediatric exclusivity" for an additional six months.
This decision is a major victory for Regeneron, as it will be able to continue to market and sell Eylea without competition from generic versions.
Source: The US Patent Trial and Appeal Board
(1).jpg)