FDA Approves Purified Cortrophin Gel Prefilled Syringe from ANI Pharmaceuticals
The US Food and Drug Administration (FDA) has approved ANI Pharmaceuticals’ Purified Cortrophin Gel (repository corticotropin injection USP) in a prefilled syringe format. This new presentation aims to simplify administration for patients requiring Cortrophin Gel therapy.
Key Details of the Approval
ANI Pharmaceuticals announced that the prefilled syringe format will be available in two single-dose options:
• 40 USP units/0.5 mL
• 80 USP units/mL
These will be accessible through Cortrophin Gel’s established specialty pharmacy network. The prefilled syringe is designed to reduce administration steps and enhance ease of use for patients.
ANI Pharmaceuticals’ Commitment to Rare Disease Portfolio
Nikhil Lalwani, President and CEO of ANI Pharmaceuticals, emphasized the company’s dedication to improving treatment accessibility and convenience.
“This approval reflects our ongoing commitment to addressing the needs of those who rely on Cortrophin Gel therapy. We look forward to making the Cortrophin Gel prefilled syringe available in the second quarter of 2025 as we continue to advance our Rare Disease portfolio.”
What is Cortrophin Gel?
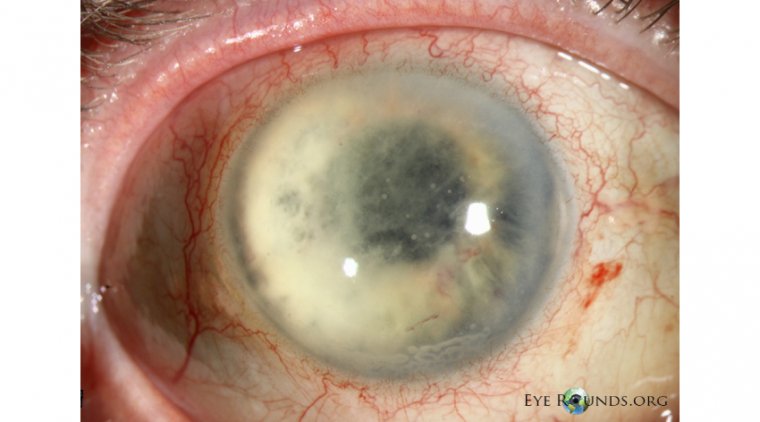
Cortrophin Gel is a prescription medication administered either subcutaneously or intramuscularly. It is indicated for severe acute and chronic allergic and inflammatory conditions affecting the eye and other parts of the body.
Ophthalmic Indications:
• Allergic conjunctivitis
• Keratitis
• Iritis and iridocyclitis
• Diffuse posterior uveitis and choroiditis
• Optic neuritis
• Chorioretinitis
• Anterior segment inflammation
Other Medical Indications:
• Acute gouty arthritis
• Rheumatoid arthritis (including juvenile rheumatoid arthritis), psoriatic arthritis, and ankylosing spondylitis
• Systemic lupus erythematosus and systemic dermatomyositis (polymyositis)
• Severe erythema multiforme (Stevens-Johnson syndrome) and severe psoriasis
• Atopic dermatitis and serum sickness
• Symptomatic sarcoidosis
• Diuresis or remission of proteinuria due to nephrotic syndrome without uremia
• Acute exacerbations of multiple sclerosis
Availability and Next Steps
ANI Pharmaceuticals plans to launch the Cortrophin Gel prefilled syringe in Q2 2025. This approval marks another step forward in enhancing treatment options for patients with rare and inflammatory diseases.
(1).jpg)