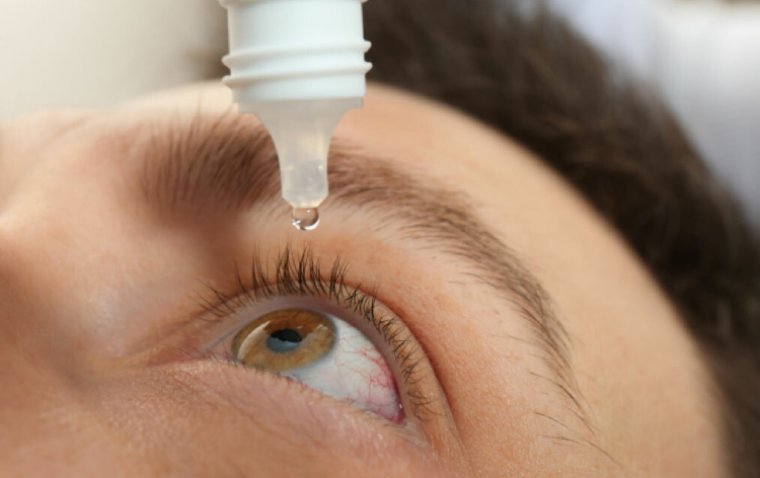
A voluntary nationwide recall of multiple ophthalmic products was issued on May 12, citing potential...
read moreOcuSciences, Inc. has announced that the U.S. Food and Drug Administration (FDA) has granted regulat...
read moreEMA has announced that the Committee for Medicinal Products for Human Use (CHMP) has issued a positi...
read more4D Molecular Therapeutics (4DMT) has announced that the U.S. Food and Drug Administration (FDA) has ...
read moreThe U.S. Food and Drug Administration (FDA) has granted Fast Track designation to OKYO Pharma for it...
read moreLuminopia has announced that the U.S. Food and Drug Administration (FDA) has expanded the label of i...
read moreRegeneron Pharmaceuticals has announced that the U.S. Food and Drug Administration (FDA) has issued ...
read moreBVI Medical has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its i...
read moreThe U.S. Food and Drug Administration (FDA) has accepted the resubmitted Biologics License Applicati...
read moreThe FDA has approved an expanded label for Iluvien, allowing its use for the treatment of chronic no...
read more More
More