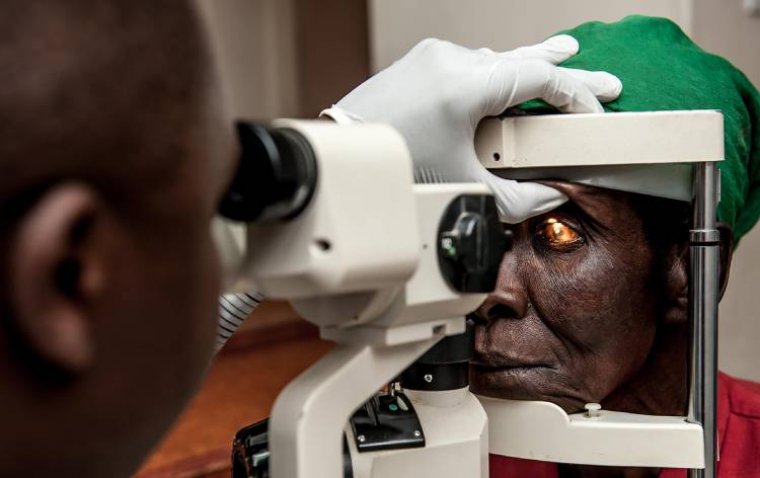
iHealthScreen has been granted a full US patent for its iPredict glaucoma detection model by the US ...
read moreENTOD Pharmaceuticals, a Mumbai-based company, has achieved a significant milestone in eye care with...
read moreEyeSouth Partners, a prominent eye care-focused management services organization, announced its newe...
read moreIn an inspiring leap towards health equity, a new eye health project in Malawi has successfully brou...
read moreCaplin Steriles Limited (Caplin), a subsidiary of Caplin Point Laboratories Limited, has received fi...
read moreMedevise Consulting, a strategic consultancy specializing in the ophthalmic sector, announced that i...
read moreOcular Therapeutix announced a significant shift in its leadership structure, as Executive Chairman,...
read moreKiora Pharmaceuticals announced that it has secured a grant from the Choroideremia Research Foundati...
read moreOasis Medical has recently unveiled its latest product, the SOFT PLUG Extended Duration 180-T Tapere...
read moreOcugen has received approval from the FDA for an amendment to their investigational new drug (IND) a...
read more More
More