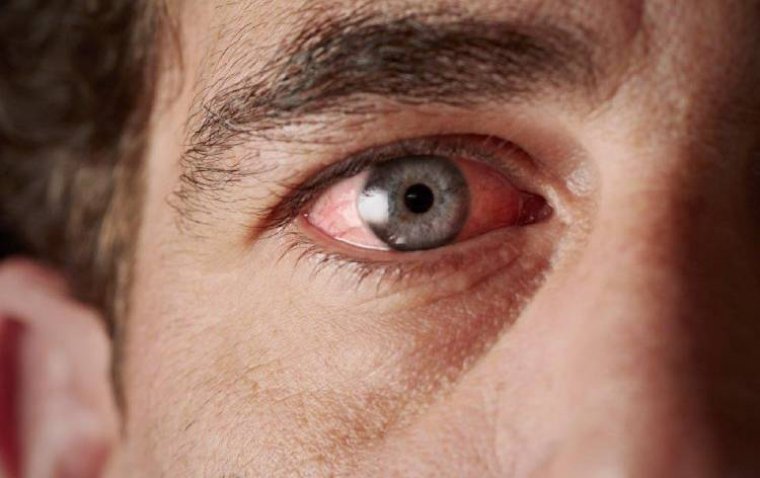
Ocular inflammation is a critical eye condition that can lead to discomfort, impaired vision, and, i...
read moreThe ability of 3D bioprinting to fabricate complex tissue constructs holds the promise of overcoming...
read moreSmoking is widely recognized as a major health hazard, contributing to a range of systemic diseases,...
read moreA Freedom of Information (FOI) request has brought to light a concerning issue: thousands of blind a...
read moreIn this article, we spotlight 10 organizations that stand out as heroes in the field of eye health, ...
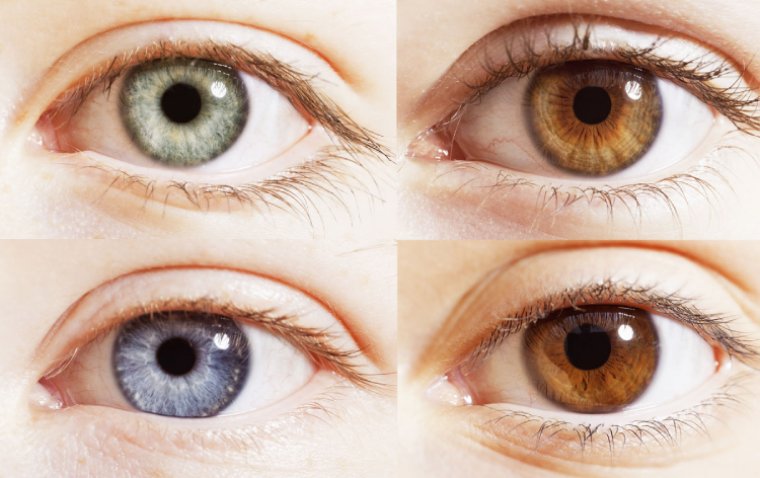
read moreEye color, a key trait that contributes to the uniqueness of an individual’s appearance, is determin...
read moreExperiencing sudden or temporary loss of vision in one eye can be a distressing event, signaling an ...
read moreIn the modern workplace, maintaining eye health has become increasingly crucial, especially as many ...
read moreDuring the period of Ramadan, questions often arise about how certain health practices, such as the ...
read moreEmerging research indicates a fascinating and complex relationship between the two, suggesting that ...
read more More
More