Verana Health has introduced its Qdata Thyroid Eye Disease (TED) module, a new addition to its suite...
read moreLEP Biomedical announced pre-seed funding of €100,000 ($107,000) over two 6-month periods, which the...
read moreSalioGen Therapeutics announced the selection of SGT-1001 as a development candidate for Stargardt d...
read morePhoenix-Micron announced a new collaboration with ArtiKode Intelligence, a pioneering artificial int...
read moreOcugen has officially completed dosing in the second cohort of its phase 1/2 ArMaDa trial of OCU410,...
read moreiHealthScreen has been granted a full US patent for its iPredict glaucoma detection model by the US ...
read moreENTOD Pharmaceuticals, a Mumbai-based company, has achieved a significant milestone in eye care with...
read moreEyeSouth Partners, a prominent eye care-focused management services organization, announced its newe...
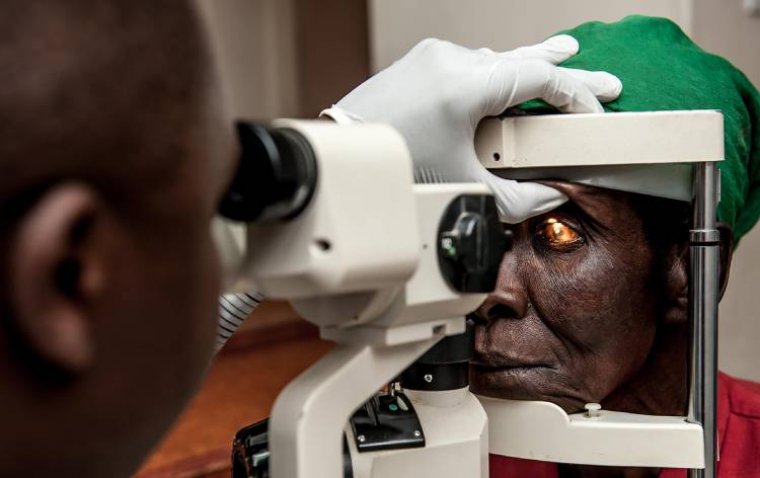
read moreIn an inspiring leap towards health equity, a new eye health project in Malawi has successfully brou...
read moreCaplin Steriles Limited (Caplin), a subsidiary of Caplin Point Laboratories Limited, has received fi...
read more More
More