The U.S. Department of Justice (DOJ) has initiated a lawsuit against Regeneron Pharmaceuticals under...
read moreGlaukos has announced the assignment of a unique, permanent J-code (J7355) by CMS for its iDose TR (...
read moreA recent study has introduced an innovative approach to treating retinal detachment through an artif...
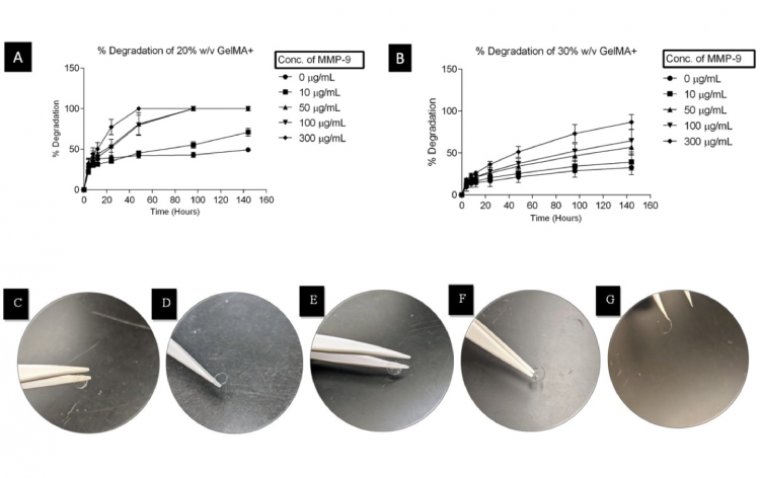
read moreWaterloo scientists have developed groundbreaking contact lenses that both protect corneal wounds an...
read moreResearch teams, under the guidance of a faculty member from Purdue University’s College of Engineeri...
read moreIn an innovative stride toward combating age-related macular degeneration (AMD), Oculogenex, Inc. ha...
read moreResearchers have unveiled an artificial intelligence (AI) technology capable of independently detect...
read moreAcuSurgical announced the successful completion of the world’s first retinal surgery performed using...
read moreThe U.S. Food and Drug Administration (FDA) has approved clobetasol propionate ophthalmic suspension...
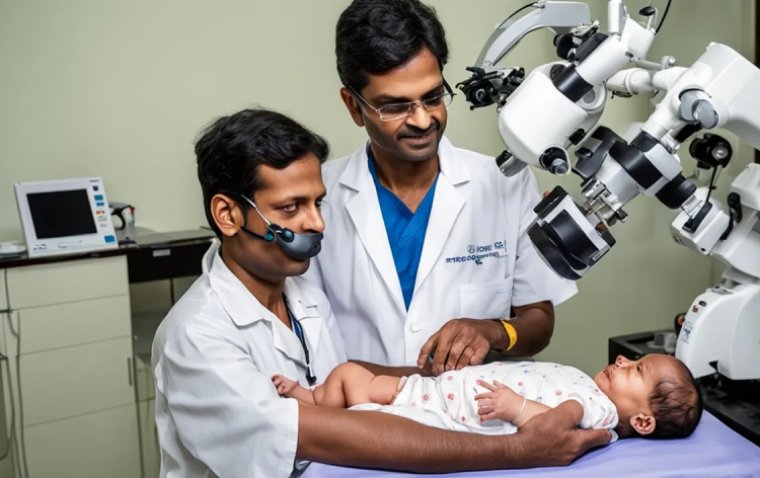
read moreNethradhama Super Speciality Eye Hospitals achieved a remarkable feat in pediatric eye care with the...
read more More
More